3D Microscaffolds for Cell biology
Scientists involved: Prof. Dr. Martin Bastmeyer, Dr. Enrico Lemma,, Magdalena Fladung, Elisa Genthner, Lucie Mißbach
Mimicking the properties of the extracellular matrix is crucial for developing in vitro models of the physiological microenvironment of living cells. 3D laser nanoprinting has emerged as a promising technology for realizing tailored 3D scaffolds for cell biology studies.
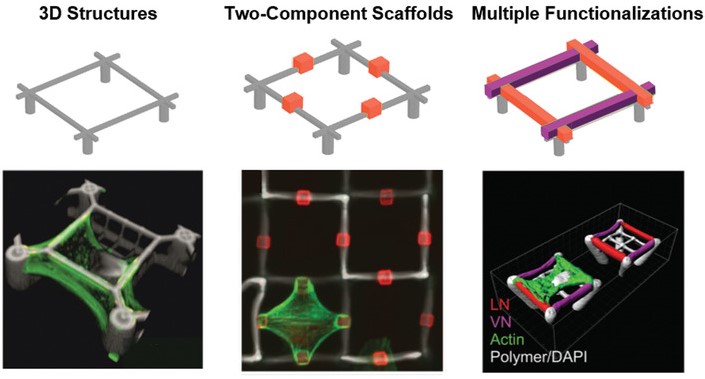
One important feature of scaffolds for 3D cell-culture is the selective functionalization of different parts of the structure. The combination of materials that allow or prevent protein adsorption makes it possible to create defined adhesion sites and control the cell shape in three dimensions. Furthermore, we employed special photoresists with specific binding groups to allow even multiple, locally defined, functionalized spots.
In addition, physical characteristics like the stiffness of the photoresists can be exploited to create a variety of different cell culture environments for studying e.g. mechanosensitive processes. For this, we are testing new photoresist for laser nanoprinting in collaboration with the groups of Prof. Eva Blasco (resists and initiators) and Prof. Christopher Barner-Kowollik (photoresists).
Apart from laser nanowriting, we employ hydrogels and 3D environments created by electrospinning to achieve control stiffness and ordering of the culture environment. These approaches are developed in collaboration with Prof. Motomu Tanaka.
Blasco Group: https://www.imseam.uni-heidelberg.de/blasco
Barner-Kowollik Group: https://www.macroarc.org/
Tanaka Group: https://www.pci.uni-heidelberg.de/bpc2/index.html
A novel approach to study cell mechanics is the use of so called metamaterials. Due to their microstructure, these materials possess properties, that go beyond those of the constituent material. Thus, they pave the way to previously inaccessible experimental conditions. In collaboration with Prof. Dr. Martin Wegener, we design different metamaterials and employ laser nanoprinting to fabricate metamaterials which are tailored to the individual experimental needs. Recently we could show in a proof-of-concept that such metamaterials can be used to alter mechanical behaviour of mesenchymal stem cells cultured on different metamaterials.
Wegener Group: https://www.aph.kit.edu/wegener/index.php
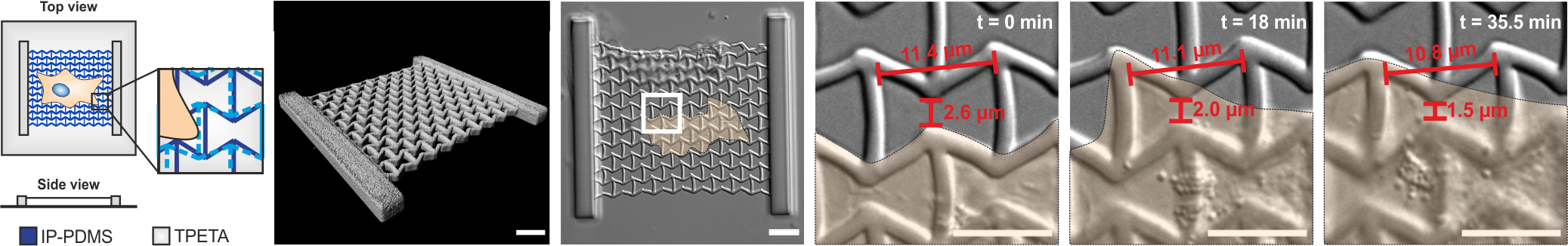
Munding*, N., Fladung*, M. et al (2023). Bio-Metamaterials for mechano-regulation of mesenchymal stem cells. Advanced Functional Materials, 2301133, https://doi.org/10.1002/adfm.202301133.
Barner-Kowollik, Christropher, Bastmeyer, Martin et al. (2017). 3D Laser Mirco- and Nanoprinting: Challenges for Chemistry. Angewandte Chemie, 56 (50), https://onlinelibrary.wiley.com/doi/full/10.1002/anie.201704695.
Hippler, Marc, Lemma, Enrico D., Bertels, Sarah et al. (2019). 3D Scaffolds to Study Basic Cell Biology. Advanced Materials, 31 (26), https://doi.org/10.1002/adma.201808110.
Richter, Benjamin, et al. (2017). Guiding cell attachment in 3D microscaffolds selectively functionalized with two distinct adhesion proteins. Advanced Materials, 29 (5), https://doi.org/10.1002/adma.201604342.
Weißenbruch, K., et al. (2022). Micro-scaffolds as synthetic cell niches: recent advances and challenges. Current Opinion in Biotechnology, 73, 290-299, https://doi.org/10.1016/j.copbio.2021.08.016.
Klein, F. et al. (2011). Two-component polymer scaffolds for controlled three-dimensional cell culture. Advanced Materials, 23 (11), 1341-1345, https://doi.org/10.1002/adma.201004060.
Lemma, E.D., et al. (2023) Selective Positioning of Different Cell Types on 3D Scaffolds via DNA Hybridization. Applied Materials & Interfaces, 15 (11), 14048-14057, https://doi.org/10.1021/acsami.2c23202.