Cell biology
Scientists involved: Dr. Kai Richler, Dr. Martina Deimling, Magdalena Fladung, Elisa Genthner, Lucie Mißbach, Prof. Dr. Martin Bastmeyer
Funding: Cluster of Excellence 3D Matter Made to Order (3DMM2O)
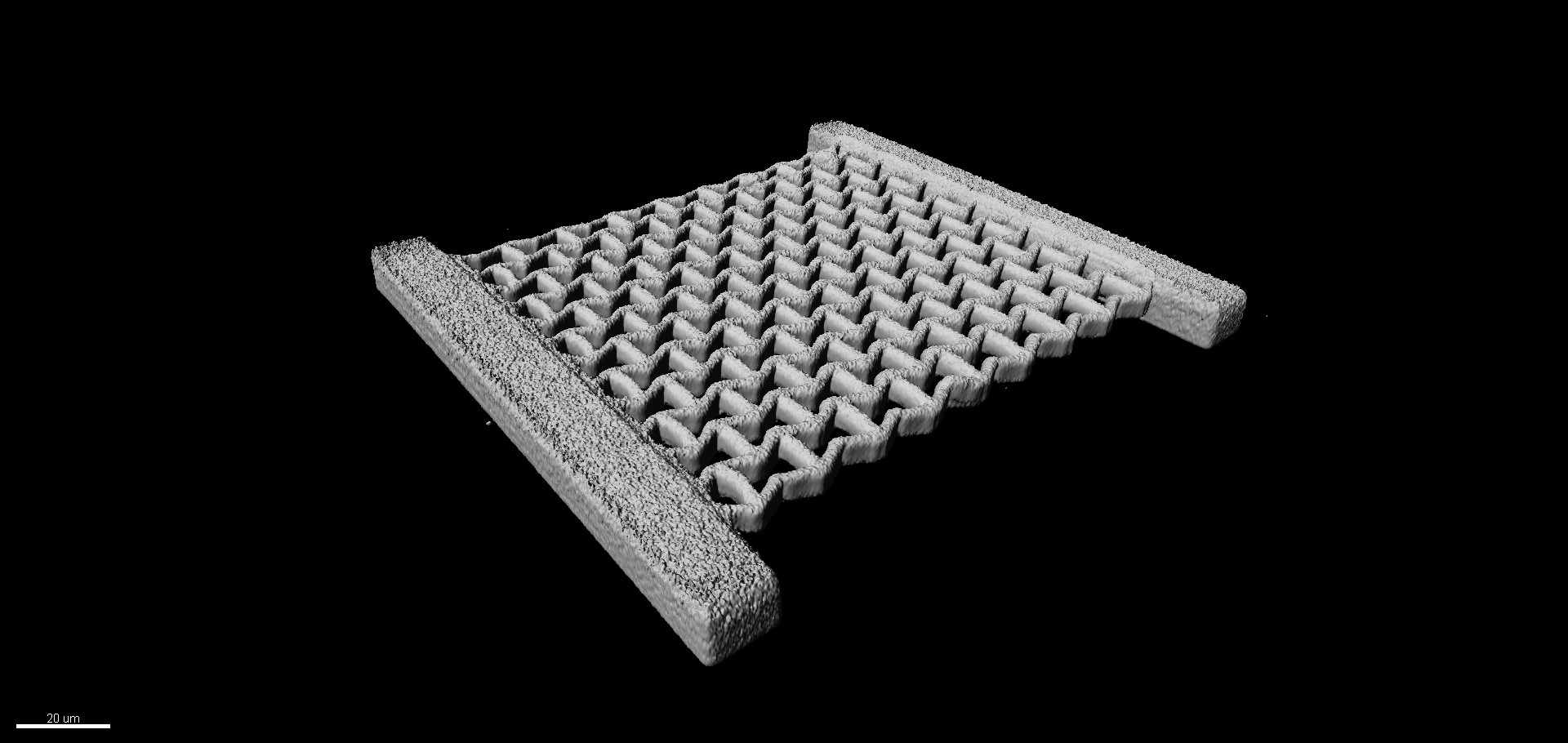
In the cell biology group, we focus on understanding the basic principles of cellular mechanobiology and developmental processes. For this, we apply a variety of models to assess the influence of environmental cues on cell fate and behaviour with the help of 3D scaffolds.
 |
 |
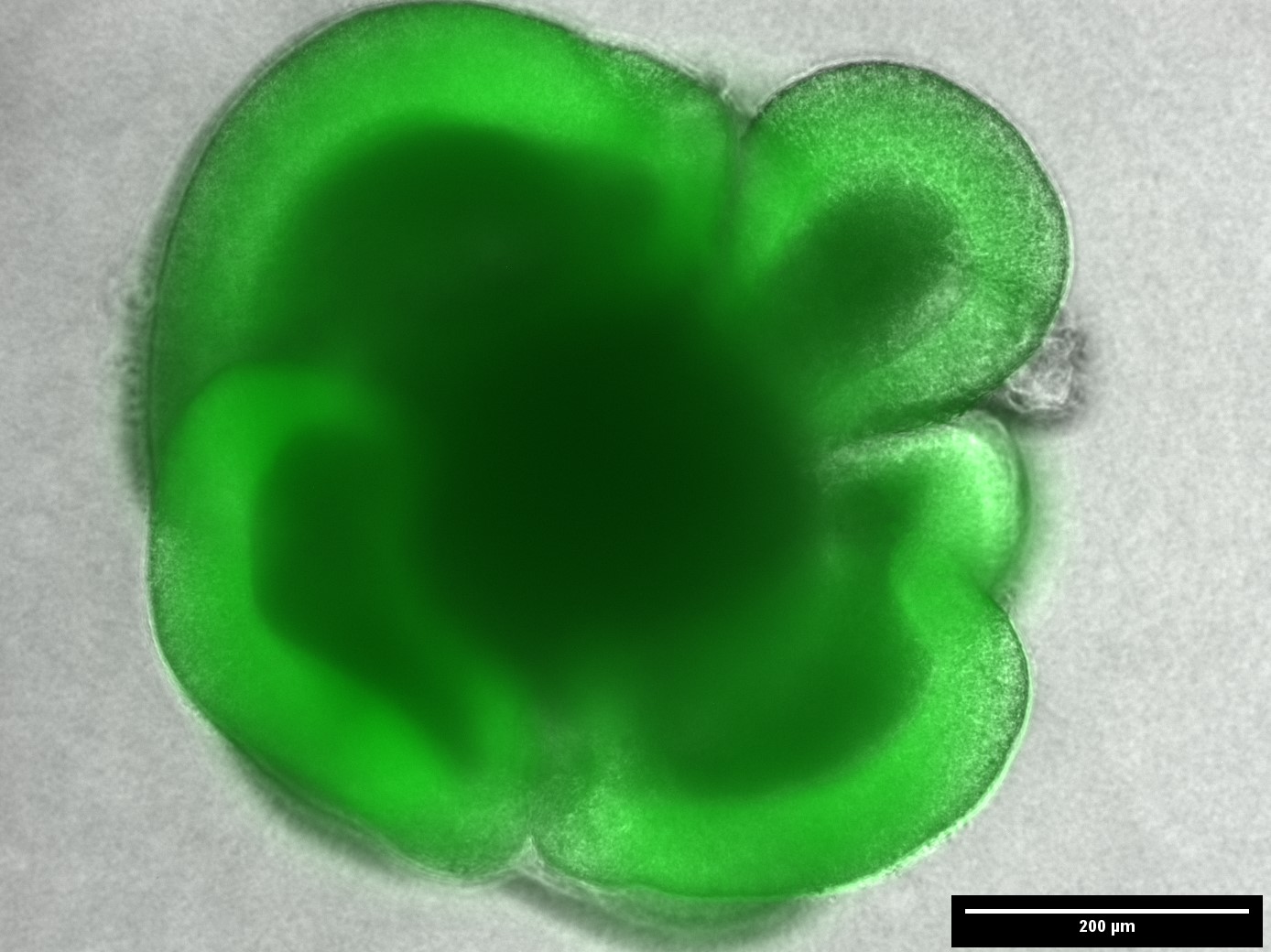 |
| 3D Microscaffolds for Cell biology | Cell Mechanics | Stem Cell Biology |