Zebrafish Neurodevelopment
Scientists involved: Dr. Joachim Bentrop, Dr. Annemarie Lange, Prof. Dr. Martin Bastmeyer
Funding: German Research Foundation (DFG)
In addition to axon guidance molecules, cell adhesion molecules (CAMs) play a major role during development of the nervous system. The spatiotemporal expression of various CAMs and their modification with glycoepitopes is involved in processes like neuronal cell migration, axonal pathfinding and target recognition. We study these processes in a simple and versatile model system, the zebrafish. Our current projects focus on cell adhesion molecules of the NCAM-type (neural cell adhesion molecule) and their modification by sialic acids. This posttranslational modification is synthesized by a family of sialyltransferases which show a highly regulated expression pattern during zebrafish development.
Besides studying gene expression patterns by in situ hybridization and immunochemistry, we apply a variety of functional assays including morpholino antisense techniques, in vivo injection of antibodies as well as transgenic expression of recombinant proteins, both, in the zebrafish and in cell culture studies. Analysis of perturbation experiments includes whole-mount immunocytochemistry with confocal laserscanning microscopy and live imaging in transgenic zebrafish lines that express green fluorescent protein (GFP) under various neuron-specific promotors.
Project 1: Phylogeny and functional divergence of NCAM homologs
Whereas other vertebrates express two NCAM paralogs, namely NCAM and OCAM, teleost fishes have duplicated the NCAM gene to yield NCAM and PCAM in addition to OCAM. To provide a molecular framework for understanding the evolution of functions in this system, we combine phylogenetic analyses, gene expression studies and functional examinations.
Project 2: Regulation and function of NCAM poly-Sialylation
Functional properties of NCAM paralogs are regulated by a unique glycan modification, namely the addition of polySialic acid. polySialic acid is known to intricately influence the homophilic and heterophilic interactions of NCAM with a multitude of cell surface and extracellular protein partners. To elucidate the mechanisms affecting NCAM polySialylation, we study the distribution of NCAM paralogs with or without polySia and the expression pattern and the enzymatic activities of enzymes of the polySia synthetic pathway.
Collaborating Scientists: Wiebke Schaper, Anja Münster-Kühnel, Rita Gerardy-Schahn (Medizinische Hochschule Hannover)
Project 3: Evolution of the polySia/NCAM - System
Towards clarifiying the conservation and/or species-specific diversification of NCAM paralogs, NCAM modifying enzymes and interactions partners, we extend our studies to farther related species, namely the Medaka model organism. In silico analyses are complemented by examining the expression patterns and the function of relevant proteins.
Collaborating Scientist: Felix Loosli (KIT)
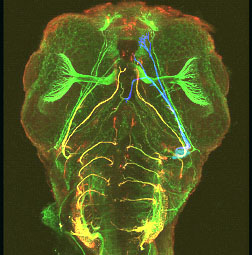
Projection pattern of cranial nerves
of a 48 hpf zebrafish wholemount embryo stained with antibodies against cell adhesion molecule zL1 (green) and polySia (red). A specific subpopulation of axons expresses polySia (yellow = overlay of green and red staining).
A subpopulation of neurons is labelled by injection of a diffusible dye (blue). (from Begemann et al., 2004)
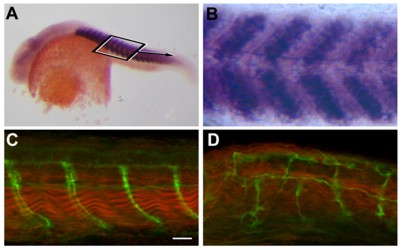
In situ hybridization shows that sialyltransferase St8SiaIII is expressed in the somites of the developing zebrafish (A) 24 phf old embryo (B) close-up of (A). (D) Morpholino-knockdown of St8SiaIII causes loosening of muscle fibrills (red) and abnormal projection of axons innverating the somites (green). (C) wild-type. (from Bentrop et al., 2008)