Stem Cell Biology
Scientists involved: Prof. Dr. Martin Bastmeyer, Dr. Kai Richler, Dr. Martina Deimling, Elisa Genthner
Development of defined 2D- and 3D-growth substrates for long-term cultivation of human induced pluripotent stem cells
Human induced pluripotent stem cells (hiPSCs) closely resemble embryonic stem cells in their developmental potential. They can differentiate into all cell types of an adult organism. Biochemical and biophysical factors of the cellular microenvironment determine which cell fate is adapted. Like human embryonic stem cells, hiPSCs grow in large, two-dimensional colonies and require Matrigel-coated culture dishes to maintain the pluripotent state.
Dissociation of hiPSCs to small aggregates or single cells inevitably leads to apoptosis. To prevent cell death different small molecule inhibitors can be applied. However, these inhibitors were shown to significantly bias cell fate decision. Furthermore, Matrigel, secreted by mouse tumor cells, is a xenogenic substance for hiPSCs with a high batch-to-batch variance and in addition is not fully characterized yet. Accordingly, Matrigel might influence hiPSC fate decision or self-renewal in an unpredictable manner.
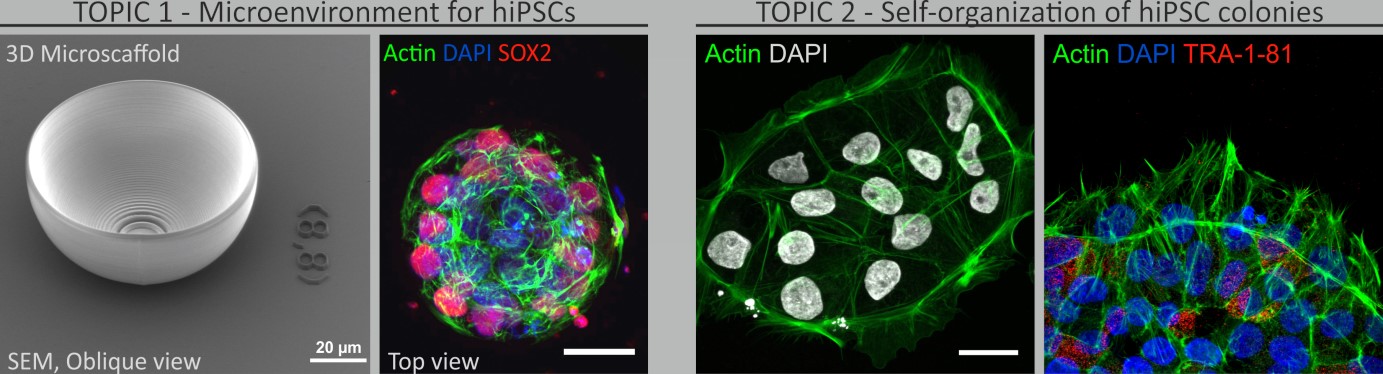
TOPIC 1: We develop well-defined protein-based surface coatings that can substitute for Matrigel in long-term hiPSC cultivation. In combination with 3D direct laser writing we design a 3D scaffold-based cell culture system that allows for the cultivation of small hiPSC aggregates and even single cells in a controllable environment.
TOPIC 2: We found that large 2D colonies consist of two populations of pluripotent cells: the border cells and the inner cells. We observed that the border cells are connected by an actomyosin ring. At present we analyze the function of the actomyosin ring for colony integrity and maintenance of self-renewal.
Further reading:
Bertels, Sarah, et al. "Geometrically defined environments direct cell division rate and subcellular YAP localization in single mouse embryonic stem cells." Scientific Reports (2021): 9269.
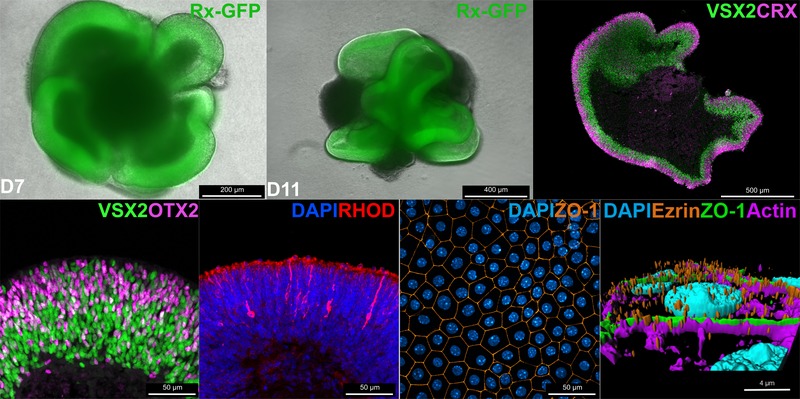
Differentiation of murine embryonic cells into retinal organoids and 3D hybrid organotypic systems
Organoids are three-dimensional in vitro models that allow the study of organ development and function. ESCs and iPSCs can be differentiated into retinal organoids which contain most of the major cell types and a similar layering found in the retina. During the differentiation protocol, organoids recapitulate fundamental processes of eye development. While the presence of many retinal cell types was already demonstrated, the functionality remains to be elucidated.
The mature retina arises from multipotent retinal progenitor cells which differentiate into all retinal cell types in a highly conserved pattern. This process requires a precise birth order of the right neurons at the right time. Eventually, post-mitotic retinal neurons need to be positioned at specific localizations to form the layering of the retina which enables the complex neuronal circuitry. In organoids, differentiation of retinal progenitor cells is highly similar to the in vivo situation which allows the investigation of this process in a scalable in vitro experiment.
The retinal pigment epithelium (RPE) is a supportive monolayer of cells in the retina which is required for proper development and maintenance of the integrity of the retina. However, in retinal organoids these cells are largely absent. RPE cells differentiated from mESCs can be grown on permeable membranes to mimic the outer blood-retina-barrier. Co-cultivation of retinal organoids and mESC-derived RPE cells allows to resemble the intimate interaction of neural retina and RPE in an in vitro system.
3D additive manufacturing allows the fabrication of scaffolding structures that enable the growth of retinal cell types in a 3D environment. The cells are intended to align on pillars to form a retina-like layering. These pillars resemble the Müller cells which span the entire thickness of the retina. This system can be combined with mESC-derived RPE cells to cultivate most of the major cell types of the retina in an artificial 3D cell culture.